Bili-ruler Evaluation
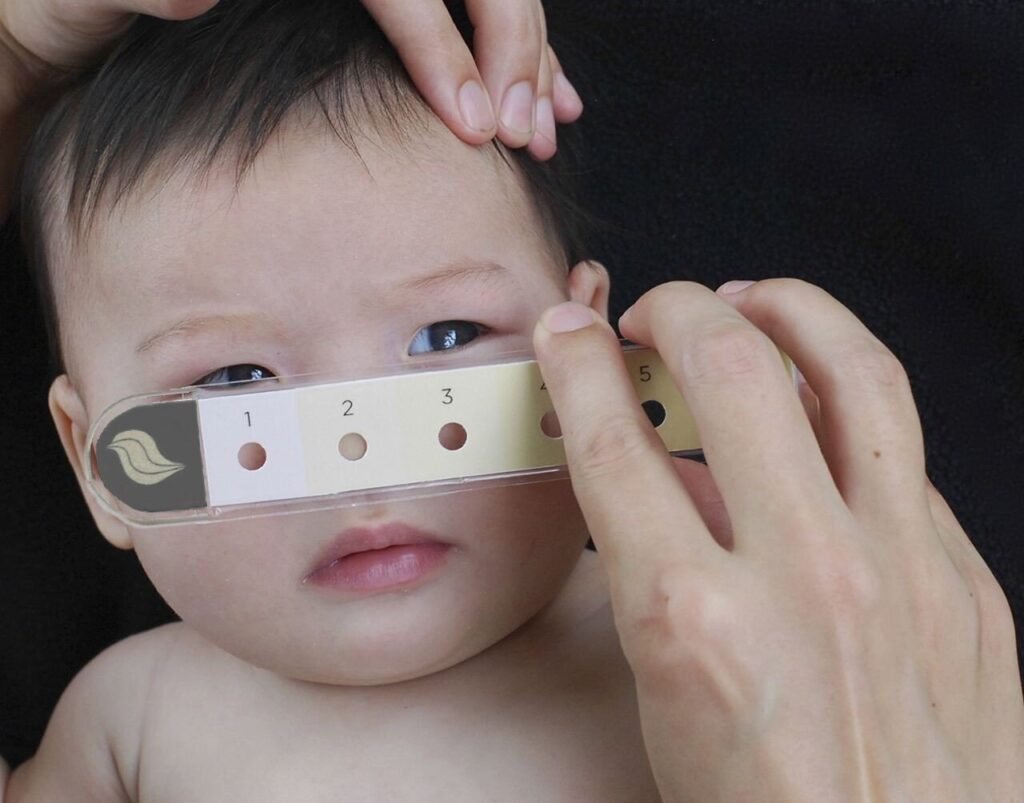
The Bili-ruler is a low-cost, easy-to-use plastic icterometer with six shades of yellow on an ordinal scale that could enable more accurate universal jaundice screening in diverse settings. This Bili-ruler evaluation is nested within the PRISMA Maternal and Newborn Health Study. Bilirubin will be assessed in newborns at 24 hours, 3 days, and 7 days postnatal. Bilirubin will be measured using visual inspection, Bili-ruler, and the BiliCare transcutaneous bilirubinometer. Skin tone will be assessed using the Monk Skin Tone Scale. The primary objectives are to assess the ability of Bili-ruler to identify significantly high bilirubin levels, as well as to assess the inter-rater agreement of Bili-ruler measurements taken by two users.
Continuous Non-invasive Hemoglobin Monitoring Device Validation Study

The Masimo Total Hemoglobin SpHb® is a continuous and non-invasive handheld device to measure hemoglobin levels. Previous research has found that SpHb is able to accurately detect hemoglobin levels in adult patients with a similar degree of bias and standard deviation to point-of-care invasive method measurements. Generally, limited clinical evidence, lack of validation of Masimo at higher than and lower than hemoglobin threshold values, and scientific consensus supporting the use of Masimo for accurate hemoglobin testing for the diagnosis of anemia during pregnancy calls for further research. We will measure hemoglobin using a venous blood sample via hematology auto-analyzer complete blood count (gold standard) and the non-invasive device to assess agreement.
